A study by Marieke Meijer (CNCR-FGA) published in EMBO journal reveals a novel and potent way for neurons to shut down synaptic transmission via tyrosine phosphorylation of Munc18-1.
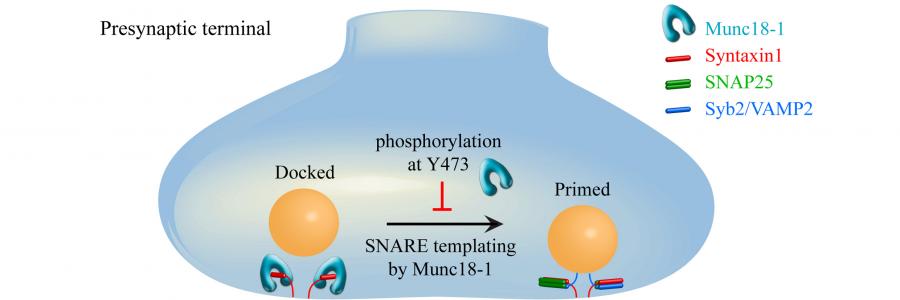
Synaptic transmission occurs within milliseconds of the arrival of an action potential. To allow for such timely precision, synapses rely on a pool of synaptic vesicles filled with neurotransmitter that are already docked at release sites and maintained in a high-energy state, known as the primed state, ready to be released. Post-translational modifications of synaptic proteins involved in synaptic vesicle release are a powerful way to modulate synaptic transmission. This study by Marieke Meijer and colleagues shows that mimicking a brain-endogenous post-translational modification on Munc18-1, a core protein in this process, specifically prevents Munc18-1 from performing its priming role. This leaves synaptic vesicles stranded at the plasma membrane, docked but not effectively primed.
The exact molecular mechanism of Munc18-1’s essential role in synaptic vesicle release has been enigmatic for decades, but recent findings have converged onto conformational changes in domain 3a, which allows Munc18-1 to form a template for the SNARE proteins that will ultimately drive vesicle release. The binding of one of the SNARE proteins, Synaptobrevin2/VAMP2, to Munc18-1 is thought to be key to this process. By collaborating with Thomas Söllner from the Heidelberg University Biochemistry Center, the authors could pinpoint the exact molecular effect of tyrosine phosphorylation of Munc18-1 to a loss of Synaptobrevin2/VAMP2 binding and a defect in the SNARE-templating function of Munc18-1.
Neuronal family members of Src family kinases are effective tyrosine kinases for Munc18-1 in heterologous cells. Src is well known for its key role in postsynaptic NMDA receptor regulation and LTP induction. However, although several Src family members are enriched at the presynapse, little is known about their physiological relevance. This study brings mechanistic insight into a novel and potent way to inhibit synapses.
Full article can be found in EMBO Journal