PhD student Javier Emperador Melero (CNCR-FGA) published his study on the role of retrograde trafficking, Golgi export and regulated secretion in Nature Communications this month. Javier is now on his way to his new job at Harvard University.
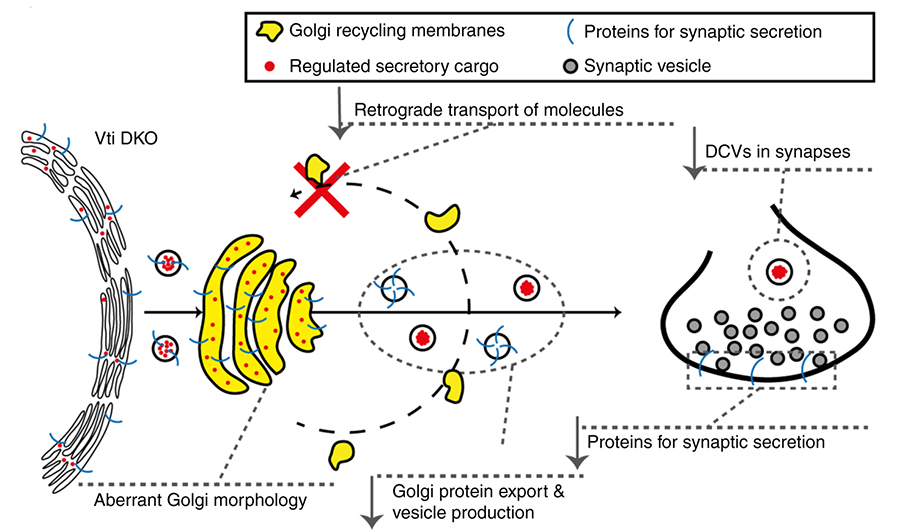
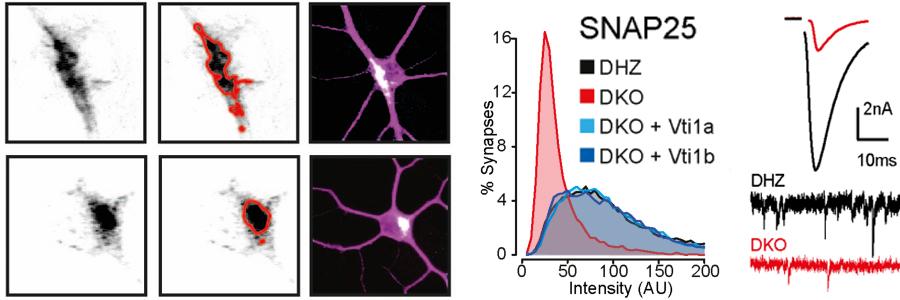
The SNARE proteins Vti1a/Vti1b have been implicated in the regulated secretion of chemical signals in the brain. However, such a role was incompatible with the established role of other SNARE proteins in secretion, especially SNAP25. Several previous studies, including several FGA studies, showed that without SNAP25 no chemical signals are released from neurons, synapses and other neurosecretory cells. The study by Emperador Melero et al. in Nature Communications of August 28th resolves this controversy. The paper shows that secretory organelles and proteins involved in regulated secretion, including SNAP25, are not efficiently delivered to the locations where they need to operate (such as synapses), when Vti1a/b function is disrupted.
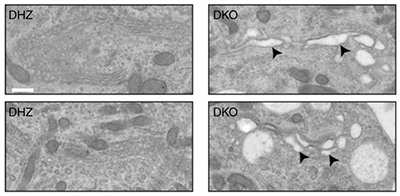
This study uncovers a new layer of regulation to support neuronal secretion and explains previously observed secretion defects in the absence of Vti1a/Vti1b. Furthermore, the study by Emperador Melero et al. shows how Vti1a/b regulates Golgi in/export to support synaptic secretion. Finally, the new study also shows that the expression of either Vti1a or Vti1b rescues virtually all cellular phenotypes in Vti1a/b deficient neurons. This challenges the current view that Vti1a and Vti1b operate in distinct pathways.
The paper is published as open access and can be viewed/downloaded here
Meanwhile, Javier has now moved on to his new job at Harvard Medical School in Boston, working as a post doc in the lab of Pascal Kaeser.