A new study by Fiona Murphy & team (FGA/MCN) on how different components of the secretion machinery work together to bind/recruit key lipids, is now published in Journal of Cell Biology.
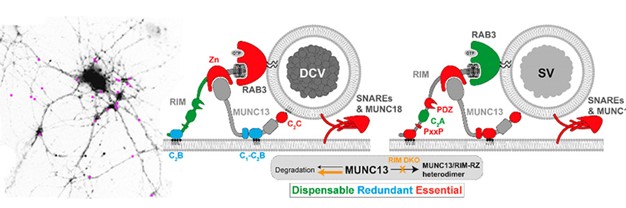
Neurons secrete chemical signal by two main principles: neurotransmitter release from synaptic vesicles (SVs) and neuropeptides from dense-core vesicles (DCVs). The presynaptic proteins RIM and MUNC13 play key roles in both pathways. However, it was still unclear how DCVs are targeted to release sites and whether RIM and MUNC13 are involved in this process. In the current study, Fiona Murphy and team show that three membrane-binding domains in RIM and MUNC13 regulate neuropeptide secretion and do so in a manner that is different from the way these same protein regulate neurotransmitter release.
Using neuropeptide secretion assays with single-vesicle resolution and peptidomics analysis of endogenous neuropeptide release in MUNC13/RIM null mutant neurons, the authors demonstrate that MUNC13 is essential for neuropeptide secretion. The N-terminus of RIM prevents MUNC13 degradation via the proteasome, and inhibiting proteasomal degradation partially restored neuropeptide secretion in RIM’s absence. RIM and MUNC13 both contain a C2 domain, a protein domain known to bind/recruit specific positively charged phospholipids in the plasma membrane (PIP2) that are known to be important for the membrane fusion reaction. The RIM C2 domain and the MUNC13 C1-C2B polybasic face are both essential for neurotransmitter release. However, the authors show that the two domains are redundant for neuropeptide secretion. In contrast, the lipid-binding MUNC13 C2C domain is essential.
This study shows that RIM and MUNC13 synergistically regulate neuropeptide secretion through membrane interactions and reveal new mechanistic differences between SV and DCV secretion principles.
The study was published in The Journal of Cell Biology and be found here: