Danai Riga
Assistant Professor
Complex behaviors that arise in response to stimulus-rich and demanding environments require the coordinated function of different brain circuits at distinct time-scales, to maintain high-order cognitive functions, such as attention, learning, memory and decision-making. Our main goal is to identify the neuromodulatory forces that mediate these processes, highlighting maladaptations that lead to aberrant cognition.
Approach or avoidance are fundamental ways we interact with the world around us, to ensure safety and meet our survival needs. The locus coeruleus (LC), the brain’s primary source of norepinephrine (NE), provides far-reaching innervation that ensures rapid and targeted NE release in response to ever-changing environments. By doing so, the LC coordinates the neurocognitive processes that guide our behavior under approach or avoidance conflicts, dictating adaptive responses to uncertainty. As such, dysregulation of the LC NE system is linked to a multitude of interrelated pathological states, including anxiety, attentional and impulse control disorders.
Collectively, our research aims to unravel the neuromodulatory influences arriving to and exerted by the LC, that ensure optimal behavioral responses to challenging environments (e.g., adversity, stress, high cognitive demand). For this, we follow two major research trajectories, investigating:
Together, we probe LC function both in terms of its regulation, i.e., LC neuromodulation by peptidergic inputs, and the consequences of that, i.e., neuromodulation of output (target) regions following NE release.
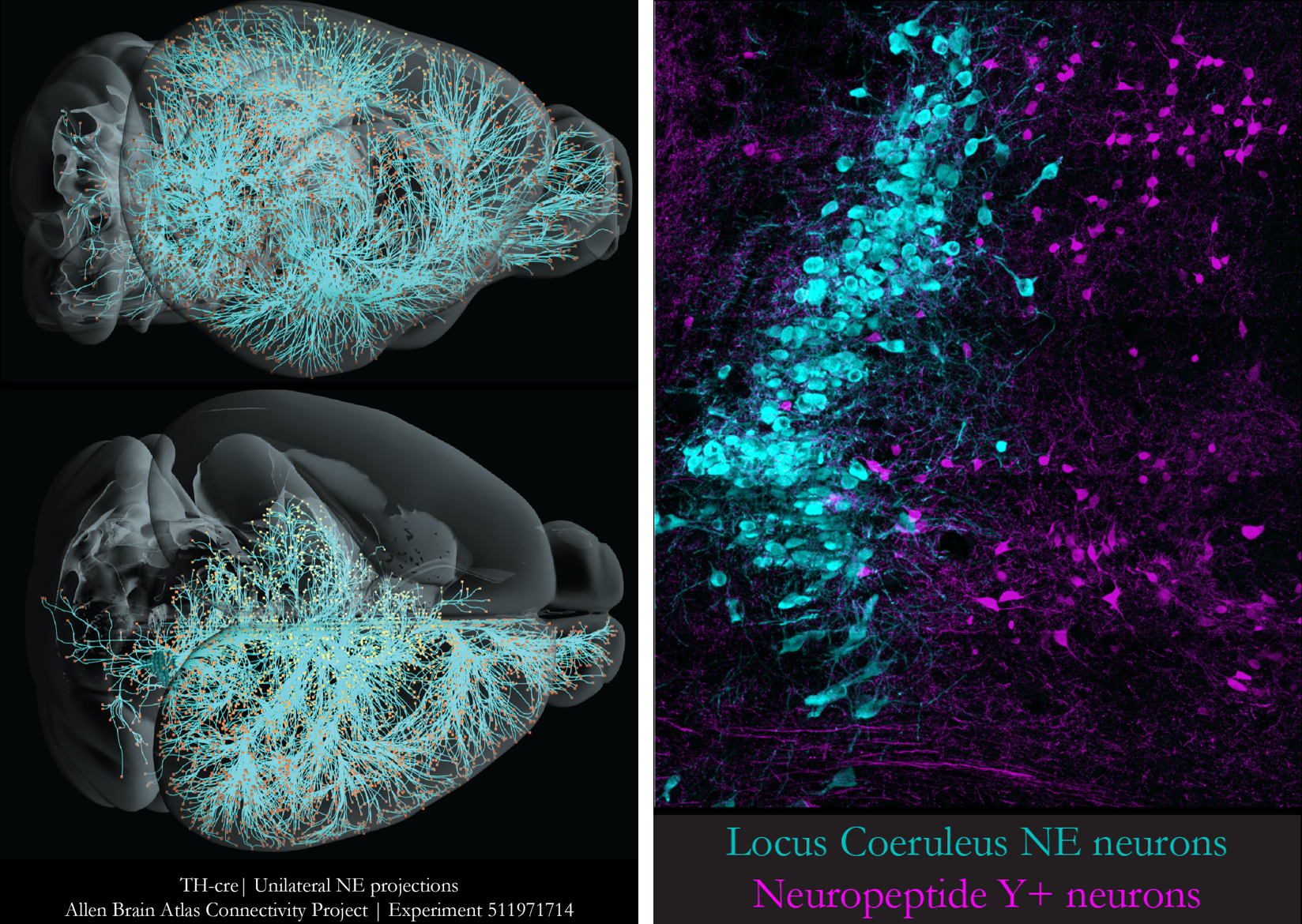
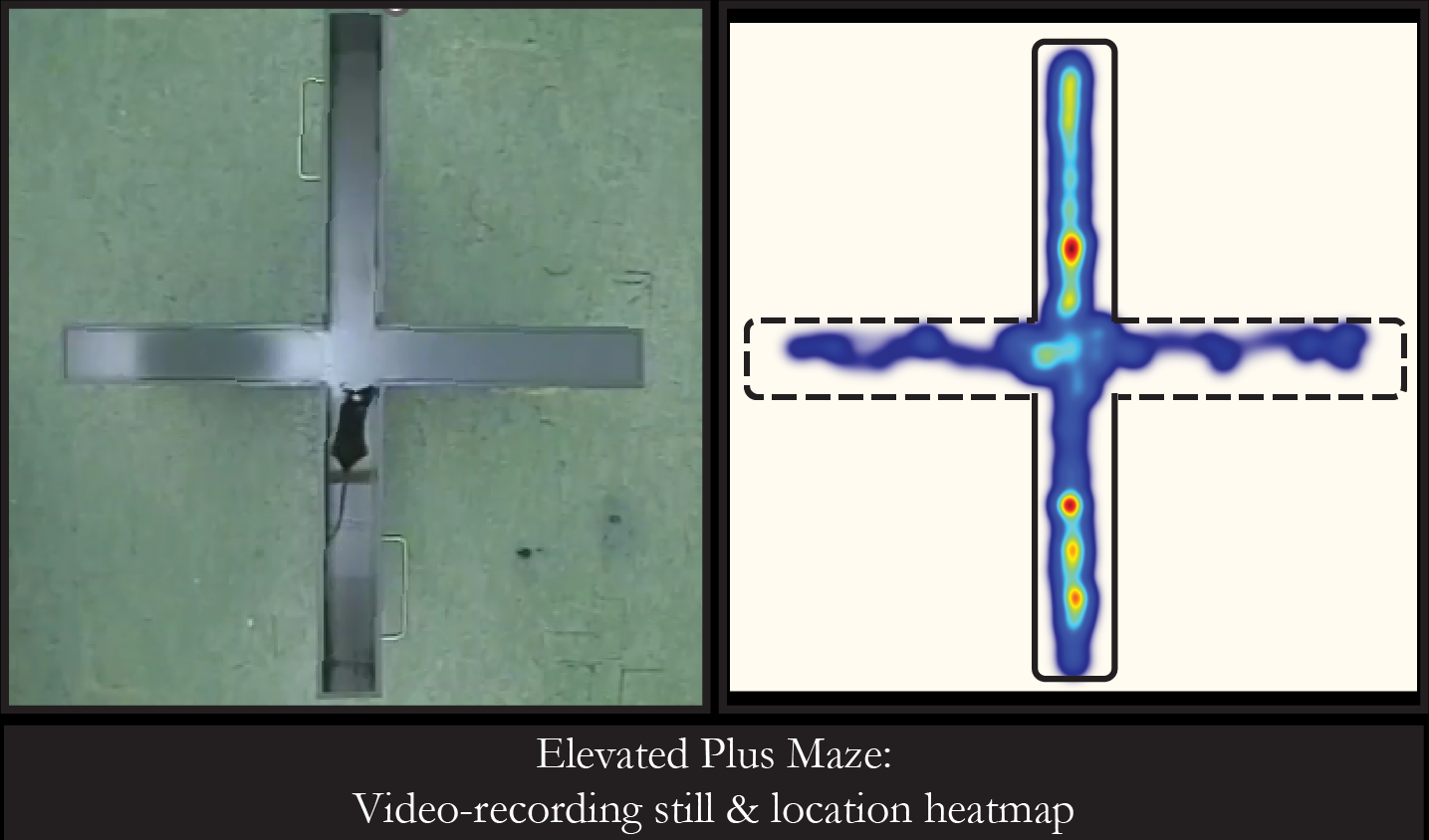
We aim to bring mechanistic insights to complex behaviors, thus, we employ a multifaceted methodological approach, that spans from the individual synapse to the intact animal. In particular, we use intersectional (viral & genetic) strategies for identification and manipulation of LC NE and/or peptidergic neuronal clusters. We complement this with opto-/chemogenetics-coupled slice electrophysiology that enables interrogation of functional connectivity in relevant circuits. Further, we employ synaptic proteomics to map the molecular architecture of the LC NE system, and its input-output landscape. Finally, we implement in-vivo imaging for monitoring NE and peptidergic dynamics, as well as in-vivo (e.g., pharmacological, pharmacogenetic) interventions to examine the causal links between neuromodulatory signalling and (mal)adaptive behaviors.
Science does not happen in a vacuum. We are proud that the team is embedded in a lively and collaborative environment, at Amsterdam Neuroscience. Here, we join forces with the teams of Dr. Rao-Ruiz (Molecular Engrams) and Dr. Michel van den Oever (Memory Circuits). In addition, we retain strong ties with the lab of Dr. Frank Meye at Utrecht UMC.