An international consortium led by Prof. Marjo van der Knaap and Prof. Frédéric Sedel (Paris) published this high impact research report on May 22 2013. The study reveals the link between autosomal-recessive CLCN2 mutations and an emerging group of leukoencephalopathies affecting brain ion and water homoeostasis, being characterised by intramyelinic oedema.
Brain white matter oedema due to ClC-2 chloride channel deficiency: an observational analytical study
Article in The Lancet Neurology
Background
Mutant mouse models suggest that the chloride channel ClC-2 has functions in ion and water homoeostasis, but this has not been confirmed in human beings. We aimed to define novel disorders characterised by distinct patterns of MRI abnormalities in patients with leukoencephalopathies of unknown origin, and to identify the genes mutated in these disorders. We were specifically interested in leukoencephalopathies characterised by white matter oedema, suggesting a defect in ion and water homoeostasis.
Methods
In this observational analytical study, we recruited patients with leukoencephalopathies characterised by MRI signal abnormalities in the posterior limbs of the internal capsules, midbrain cerebral peduncles, and middle cerebellar peduncles from our databases of patients with leukoencephalopathies of unknown origin. We used exome sequencing to identify the gene involved. We screened the candidate gene in additional patients by Sanger sequencing and mRNA analysis, and investigated the functional effects of the mutations. We assessed the localisation of ClC-2 with immunohistochemistry and electron microscopy in post-mortem human brains of individuals without neurological disorders.
Findings
Seven patients met our inclusion criteria, three with adult-onset disease and four with childhood-onset disease. We identified homozygous or compound-heterozygous mutations in CLCN2 in three adult and three paediatric patients. We found evidence that the CLCN2 mutations result in loss of function of ClC-2. The remaining paediatric patient had an X-linked family history and a mutation in GJB1, encoding connexin32. Clinical features were variable and included cerebellar ataxia, spasticity, chorioretinopathy with visual field defects, optic neuropathy, cognitive defects, and headaches. MRI showed restricted diffusion suggesting myelin vacuolation that was confined to the specified white matter structures in adult patients, and more diffusely involved the brain white matter in paediatric patients. We detected ClC-2 in all components of the panglial syncytium, enriched in astrocytic endfeet at the perivascular basal lamina, in the glia limitans, and in ependymal cells.
Interpretation
Our observations substantiate the concept that ClC-2 is involved in brain ion and water homoeostasis. Autosomal-recessive CLCN2 mutations cause a leukoencephalopathy that belongs to an emerging group of disorders affecting brain ion and water homoeostasis and characterised by intramyelinic oedema.
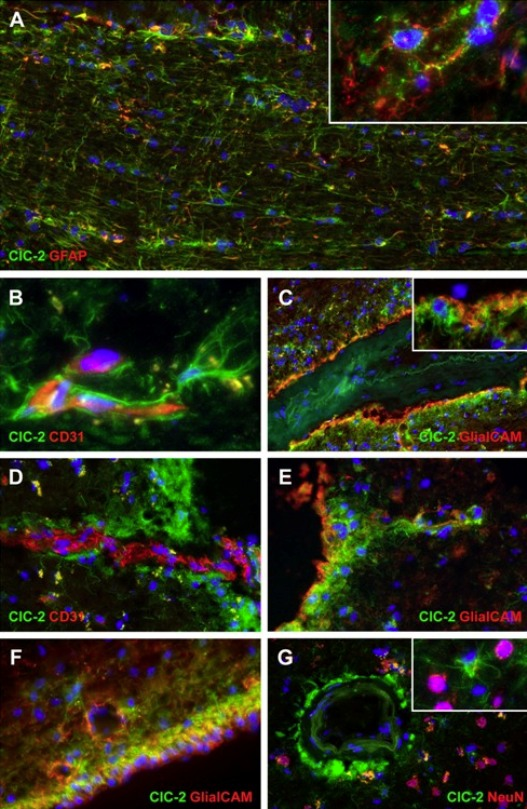
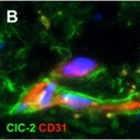
Figure 3: Expression of ClC-2 in a healthy adult human brain
Double immunohistochemical staining of the cerebral white matter for ClC-2 (green) and the astrocyte-specific marker GFAP (red) shows that all GFAP-positive astrocytes express ClC-2 (A). Note the long ClC-2-positive astrocytic processes extending parallel and perpendicular to the direction of the fibre bundle. ClC-2 shows a punctate immunoreactivity, as expected for a membrane protein (inset in A). Colabelling of the same tissue for ClC-2 (green) and the vascular endothelial cell marker CD31 (also known as PECAM1; red) shows that expression of ClC-2 is enhanced in the astrocytes surrounding the blood vessels deep in the parenchyma (B) and the penetrating vessels at the surface of the brain (D). Note the many fine astrocytic processes running along the white matter capillary (B). Perivascular astrocytes ©, subpial astrocytes (E), and ependymal cells (F) coexpress GlialCAM (red) with ClC-2 (green), but GlialCAM immunoreactivity extends further into the astrocytic processes and endfeet (inset in C). Double staining of the frontal cortex for ClC-2 (green) and the neuronal marker NeuN (red) shows that, in this location, ClC-2 immunoreactivity is restricted to perivascular astrocytes (G). No ClC-2 expression is seen in the NeuN-positive neuronal cells (inset in G). In all images the cell nuclei are stained with DAPI (4´,6-diamidino-2-phenylindole; blue).